Sterile packaging material—Sterile wrap, crepe paper, sterile pouch together for the sterile barrier
- Categories:Press
- Author: Peter.hu
- Origin: Sengong
- Time of issue:2021-07-07
- Views:0
(Summary description)According to the ISO 11607-1:2006 Final Sterilized Medical Device Packaging Part 1, the standard specifies specific requirements for materials, sterile barrier systems, and packaging systems.
At the same time, EN868-2~EN868~10 "packaging materials and system standards for medical devices to be sterilized" also explain the packaging of medical devices.
At present, ISO11607 and EN868 are internationally recognized standard systems involving the final sterilization of medical device packaging.
Basic concept of packaging
ISO 11607-1 uses the term "sterile barrier system" to describe the initial packaging of ultimately sterilized medical devices after harmonizing international medical device packaging terms.
The integration of the sterile wrap and pouch used for medical device packaging produced by Nanjing Sengong BioTechnology Co., Ltd. are called "preformed aseptic barrier system".
As a special case, aseptic liquid path packaging designed to ensure that the device is partially sterile by the tightness of sealing devices such as protective sleeves and devices to ensure that the device is expected to come into contact with the liquid is also considered sterile barrier systems.
The packaging system usually consists of two parts: sterile barrier system and protective packaging.
The sterile barrier system is a basic sterile barrier for the final sterilization of medical devices.
Protective packaging is secondary packaging that provides additional protection for a sterile barrier system.
At present, the commonly used sterile barrier system can be divided into reusable sterile barrier system and disposable sterile barrier system.
Reusable sterile barriers include reusable cotton cloth, and sterilizing device cases.
Disposable sterile barrier includes non-woven fabric, crepe paper, sterile pouch and other disposable sterilization materials.
The following are descriptions of several materials
1. The combination of preformed chassis and cover packaging, this type of packaging chassis is usually preformed by hot forming or pressing molding process.
The cover (having a fixed shape) can be breathable or airtight, typically with a heat sealing layer that can heat seal the cover on the chassis.
This form of packaging is commonly used for larger and heavier instruments, such as orthopedic implants and pacemakers and surgical boxes.
Sterile packaging material—Sterile wrap, crepe paper, sterile pouch together for the sterile barrier
(Summary description)According to the ISO 11607-1:2006 Final Sterilized Medical Device Packaging Part 1, the standard specifies specific requirements for materials, sterile barrier systems, and packaging systems.
At the same time, EN868-2~EN868~10 "packaging materials and system standards for medical devices to be sterilized" also explain the packaging of medical devices.
At present, ISO11607 and EN868 are internationally recognized standard systems involving the final sterilization of medical device packaging.
Basic concept of packaging
ISO 11607-1 uses the term "sterile barrier system" to describe the initial packaging of ultimately sterilized medical devices after harmonizing international medical device packaging terms.
The integration of the sterile wrap and pouch used for medical device packaging produced by Nanjing Sengong BioTechnology Co., Ltd. are called "preformed aseptic barrier system".
As a special case, aseptic liquid path packaging designed to ensure that the device is partially sterile by the tightness of sealing devices such as protective sleeves and devices to ensure that the device is expected to come into contact with the liquid is also considered sterile barrier systems.
The packaging system usually consists of two parts: sterile barrier system and protective packaging.
The sterile barrier system is a basic sterile barrier for the final sterilization of medical devices.
Protective packaging is secondary packaging that provides additional protection for a sterile barrier system.
At present, the commonly used sterile barrier system can be divided into reusable sterile barrier system and disposable sterile barrier system.
Reusable sterile barriers include reusable cotton cloth, and sterilizing device cases.
Disposable sterile barrier includes non-woven fabric, crepe paper, sterile pouch and other disposable sterilization materials.
The following are descriptions of several materials
1. The combination of preformed chassis and cover packaging, this type of packaging chassis is usually preformed by hot forming or pressing molding process.
The cover (having a fixed shape) can be breathable or airtight, typically with a heat sealing layer that can heat seal the cover on the chassis.
This form of packaging is commonly used for larger and heavier instruments, such as orthopedic implants and pacemakers and surgical boxes.
- Categories:Press
- Author: Peter.hu
- Origin: Sengong
- Time of issue:2021-07-07
- Views:0
According to the ISO 11607-1:2006 Final Sterilized Medical Device Packaging Part 1, the standard specifies specific requirements for materials, sterile barrier systems, and packaging systems.
At the same time, EN868-2~EN868~10 "packaging materials and system standards for medical devices to be sterilized" also explain the packaging of medical devices.
At present, ISO11607 and EN868 are internationally recognized standard systems involving the final sterilization of medical device packaging.
Basic concept of packaging
ISO 11607-1 uses the term "sterile barrier system" to describe the initial packaging of ultimately sterilized medical devices after harmonizing international medical device packaging terms.
The integration of the sterile wrap and pouch used for medical device packaging produced by Nanjing Sengong BioTechnology Co., Ltd. are called "preformed aseptic barrier system".
As a special case, aseptic liquid path packaging designed to ensure that the device is partially sterile by the tightness of sealing devices such as protective sleeves and devices to ensure that the device is expected to come into contact with the liquid is also considered sterile barrier systems.
The packaging system usually consists of two parts: sterile barrier system and protective packaging.
The sterile barrier system is a basic sterile barrier for the final sterilization of medical devices.
Protective packaging is secondary packaging that provides additional protection for a sterile barrier system.
At present, the commonly used sterile barrier system can be divided into reusable sterile barrier system and disposable sterile barrier system.
Reusable sterile barriers include reusable cotton cloth, and sterilizing device cases.
Disposable sterile barrier includes non-woven fabric, crepe paper, sterile pouch and other disposable sterilization materials.
The following are descriptions of several materials
1. The combination of preformed chassis and cover packaging, this type of packaging chassis is usually preformed by hot forming or pressing molding process.
The cover (having a fixed shape) can be breathable or airtight, typically with a heat sealing layer that can heat seal the cover on the chassis.
This form of packaging is commonly used for larger and heavier instruments, such as orthopedic implants and pacemakers and surgical boxes.
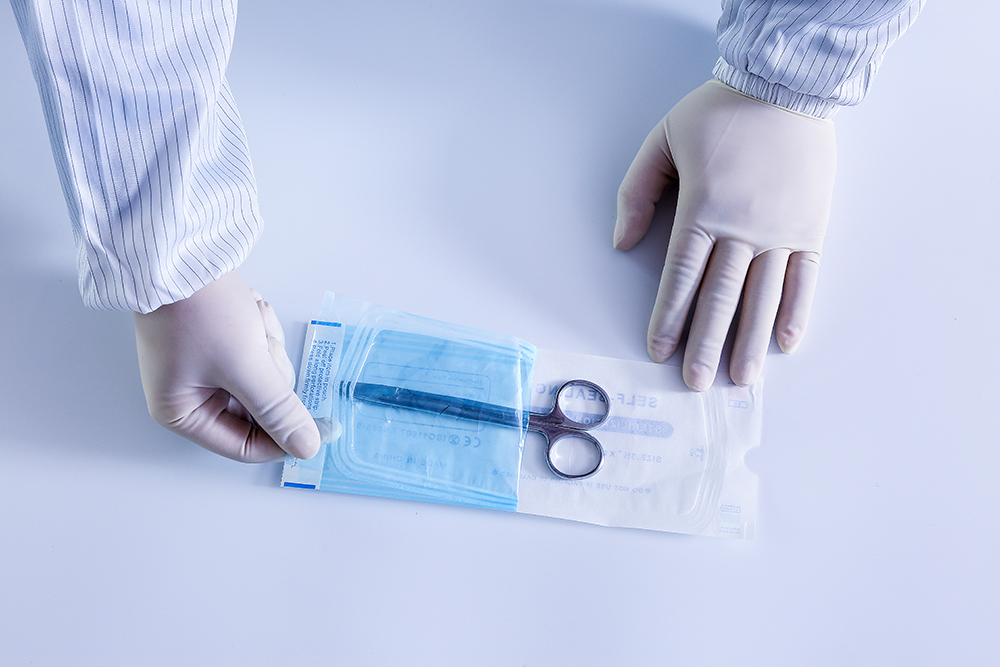
2. Soft and removable composite bag. The typical structure of the composite bag is that one side is membrane and the other side is membrane, paper or nonwovens.
The pouches are often supplied in the form of preformed sterile barrier systems.
All seals have been formed except for an opening (usually at the bottom). The reserved opening is for re-sealing after loading the device. Because the width of the pouch can be processed to a variety of specifications, the pouch can be used as a sterile barrier system for a variety of devices. These instruments are characterized by their small size and light weight. Pouches can have different design features.
For example, it can be a folded bag (also known as a solid bag), which fits into a thicker instrument, or it can be a flat bag. The purpose of the membrane is to see the instrument inside before reopening, and the purpose of the paper is to allow the sterilizer (steam, ethylene oxide gas) into the package.
3. Sterilization paper bag, sterilization paper bag has only one kind of medical grade porous manufacturing, by folding the growth of the cylinder. The drum is sealed with two lines of glue along its length, then cut to the required specifications, one end is sealed with one or more layers of adhesive, and folded many times to improve the sealing strength. The open end is usually designed with a wrong side or a thumb cut to facilitate separating the paper bag when loading the device. The final closure of the bag is formed prior to sterilization.

4. Head bag. The head bag consists mainly of two airtight but mutually compatible film surfaces, one of which is usually left several inches to be integrated with the gummed breathable material.
The breathable material can be used to peel the body of the bag for final use. The head bag is mainly used to hold large volume items, such as equipment bags.
5, Forming/Filling/Sealing (FFS) process packaging, the sterile barrier system is produced in three in one production (e.g., automated production line), there is a combination of bag form, forming the chassis and cover of the combination form, can also have a soft bottom film surface pressed into a certain shape. In the process of three-in-one production, the upper and lower package parts are put into the FFS machine. The working procedure of the machine is to shape the lower package first, then load the instrument and cover the upper package part, and finally seal to form a sterile barrier system.
The above specific explanation of the sterilization packaging material products, Nanjing Sengong can provide the above sterilization packaging products, have passed a series of tests such as ISO13485, CE, ISO1607, EN868, ISO10993, has a rich experience in the production of sterilization packaging materials used in the disinfection supply room, customization available according to customer.
Scan the QR code to read on your phone
Contact information

B8-201,NO.9 Kechuang Avenue,jiangbei new area, Nanjing City




